Professor Chia-Ying Chiang’s team from Taiwan Tech boosts glycerol value with innovative electrolyte adjustments!
Professor Chia-Ying Chiang from the Department of Chemical Engineering at Taiwan Tech, in collaboration with Professor Tomohiro Hayashi's team from the Tokyo Institute of Technology, discovered that adjusting the glycerol-borate ratio can enhance the production of high-value dihydroxyacetone (DHA), featured in the October issue of the international journal “Journal of Catalysis”.
Professor Chia-Ying Chiang's team from the Department of Chemical Engineering at Taiwan Tech found that simply adjusting the concentration ratio of glycerol and the auxiliary electrolyte (borate component) can significantly enhance the value of biodiesel waste (glycerol).
Professor Chia-Ying Chiang's research team at Taiwan Tech utilized widely-used nickel electrodes commonly used in the industry to conduct high-value transformation reactions of biodiesel waste glycerol and green hydrogen production experiments through water decomposition. By simply adjusting the concentration ratio of glycerol and the auxiliary electrolyte (borate component), they significantly increased the yield of dihydroxyacetone (DHA). DHA is a high-value product worth hundreds of dollars per kilogram, primarily used in cosmetics, pharmaceuticals, and food industries; for instance, “dihydroxyacetone” is a key ingredient in sun aid products.
Chiang noted that the biodiesel industry generates nearly 10-20% waste, primarily glycerol. Transforming glycerol into economically valuable products to enhance the biodiesel industry's profitability and promote sustainable energy development has been a long-standing research focus. Previous studies primarily concentrated on solid catalysts; her team had also researched copper oxide as a catalyst material, published in the international journal “Applied Catalysis B: Environmental” in 2020.
However, this study revealed that borate ions in the solution interact with glycerol, suggesting that the electrolyte composition is crucial. Additionally, in hydrogen production via water electrolysis, there is a risk of explosion if hydrogen and oxygen gases are not separated by a membrane. However, by adjusting the glycerol-borate ratio appropriately, the team was able to suppress the oxidation reactions that produce oxygen at the anode, ensuring that hydrogen and oxygen do not simultaneously generate dangerous conditions in the reactor. This significantly enhances safety.
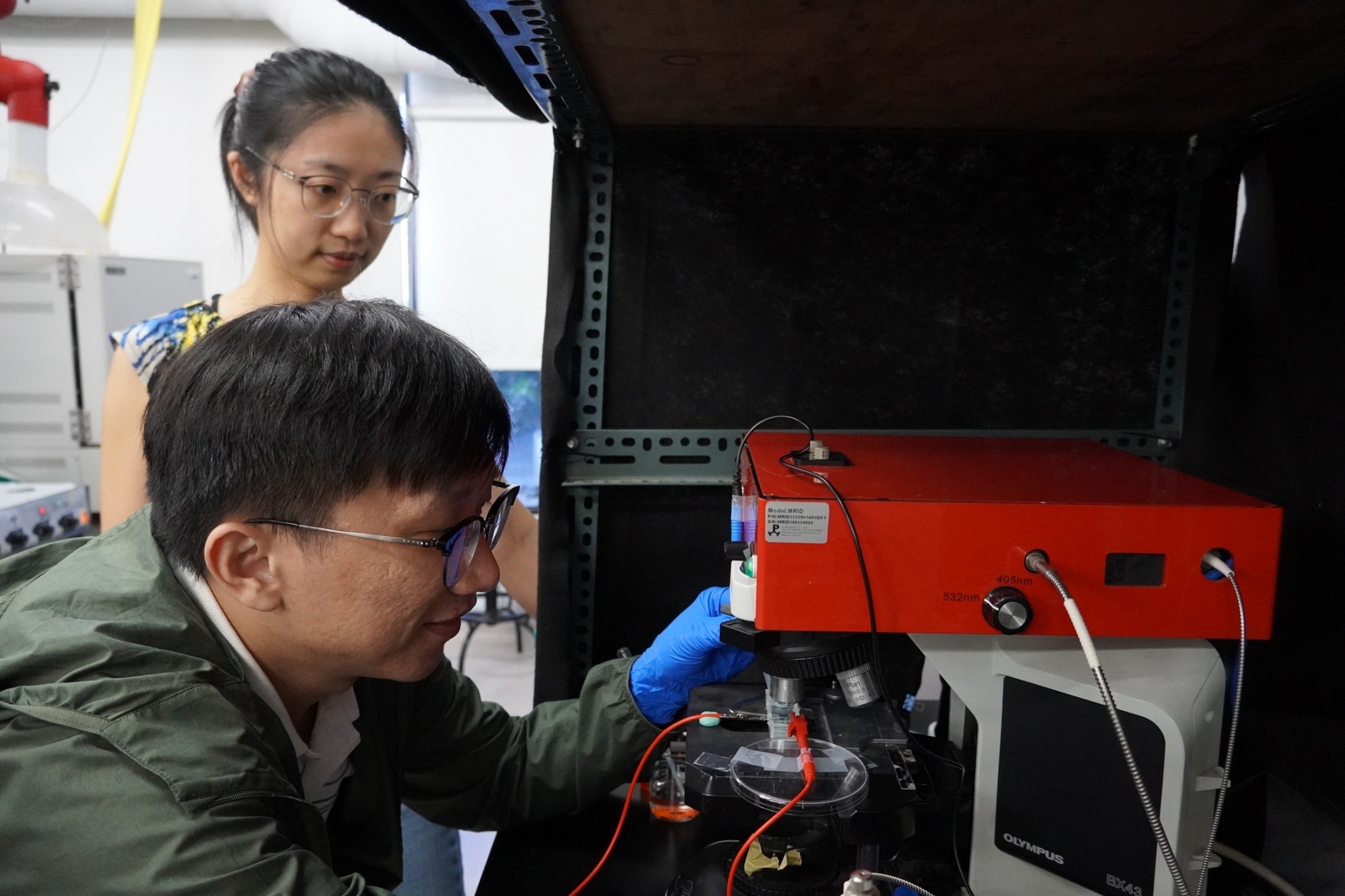
Team member postdoctoral fellow Giang-Son Tran (front) conducts glycerol reactions, utilizing in-situ Raman spectroscopy to monitor real-time changes in electrode materials and solution composition. Behind is Professor Chia-Ying Chiang.
In addition, the team switched to conducting experiments using “nickel oxide”, a catalyst for hydrogen production in water electrolysis that does not contain precious metals. This component is not a high-cost material, so it can be widely used in the industry. At the same time, because the integrated system does not produce hydrogen and oxygen at the same time, and there is no need for a separator between the two electrodes to avoid the risk of explosion, it can reduce the energy loss of the system and produce green hydrogen and “dihydroxyacetone” more efficiently and energy-savingly.
Chia-Ying Chiang said that thanks to the National Science Council’s project subsidy and the support of the Japan-Taiwan Exchange Association's “Joint Research Funding Program”, this research and development result will allow the industry to process waste glycerol with minimal energy and the lowest cost, and produce high-priced products “dihydroxyacetone” and green hydrogen. The team plans to build on the results of this research and development, and further design new types of electrochemical reactors suitable for large-scale manufacturing processes in the industry, aiming to transition the concepts of green hydrogen production and high-value organic waste conversion from the lab to real-world applications, allowing the green energy and biomass energy industries to sustainable and self-sufficient healthy development.

Team member Dr. Giang-Son Tran conducted electrochemical experiments for glycerol oxidation reactions.
